
Bringing Radioligand Therapy (RLT) Clinical Trials to Your Fingertips
Featured Sponsor



Featured News In Theranostics
More Stories

Genetic mutations linked to poor outcomes in Pluvicto patients
Mon Feb 23 2026

ICPO and UroTeragLATAM signed a strategic agreement to accelerate Theranostics for urological cancer in Latin America
Fri Feb 27 2026

Twist Bioscience Expands Antibody Discovery Offering with Bispecific Licensing Agreement
Tue Feb 17 2026
Podcasts
FDA Approval of 177Lu-PSMA-617 for Taxane-Naive mCRPC - Oliver Sartor
PSMA DC Trial: Lutetium + SBRT to Delay ADT in Oligometastatic Prostate Cancer - Oliver Sartor
Considerations for Patient Selection and Imaging in PSMA-Targeted Therapy - Tanya Dorff & Julie Graff
Why TheranosticTrials.org?
The leading global platform for radiopharmaceutical & molecular imaging clinical trials
Global Reach
We are uniquely positioned at the heart of Theranostics.
Increasing Awareness
We're dedicated to increasing awareness about Theranostics and it's potential.
Fostering Engagement
We encourage active participation in the Theranostics community.
Building Connections
We connect physicians, clinics, and patients with Theranostic opportunities.
Comprehensive Resources
Access a complete list of cancer Theranostic Trials and educational materials.
Advancing the Field
We collaborate with key opinion leaders to push Theranostics forward.
Our Ultimate Goal
Provide more hope to cancer patients everywhere!
Navigating Tips
Pick Your Path
Stats
Theranostic Trials Global Ecosystem
View All


Distinguished Investigator
A Distinguished Investigator of Theranostics is a level of distinction conferred upon an individual physician who has demonstrated a mastery of conducting novel radioligand therapies and molecular imaging clinical trials.
RLT Components
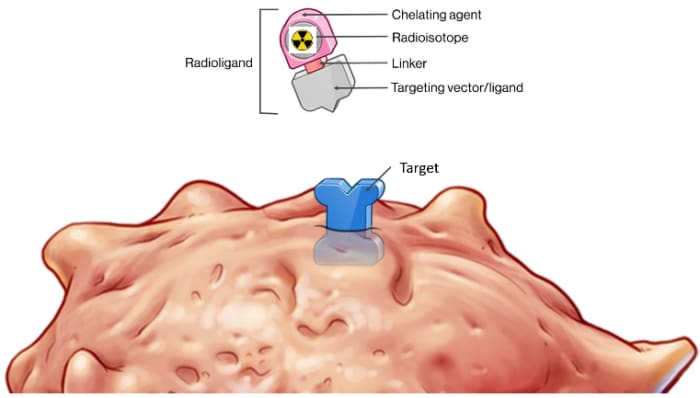
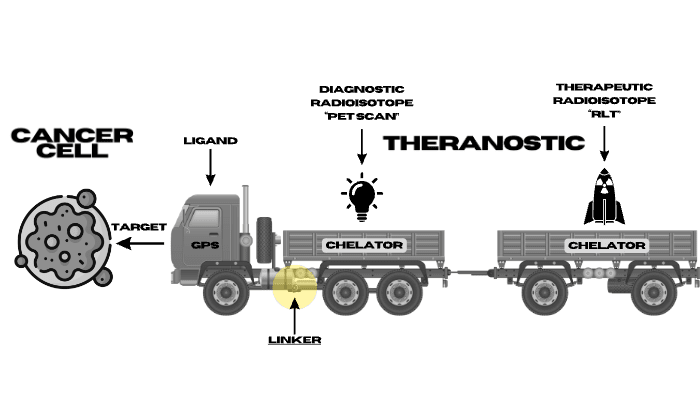
RADIOLIGAND THERAPY (RLT) is a highly effective approach to very accurately locating cancer cells and effectively killing those same cells by delivery various radioisotopes to a specific target that is located on a type of cancer. Some of the Radioisotopes are diagnostic for locating cancers & some therapeutic to treat the cancers.
There are several Components to a RLT that are simply demonstrated in the truck diagram including:
- Cancer Targets
- Ligand (demonstrated as the Truck GPS set to find a specific Target)
- Diagnostic Radioisotopes (demonstrated as light bulbs that light the cancer Targets on a PET scan)
- Therapeutic Radioisotopes (demonstrated as bombs that kill cancer cells that express the target with either Alpha or Beta radiation)
- Linker (demonstrated as the hitch keeping the Radioisotope attached to the Ligand)
- Chelator (demonstrated as the Trailers which keeps the Radioisotope on Target).
To learn more about the specific components being studied today on clinical trials around the world check out the RLT COMPONENTS Tab.
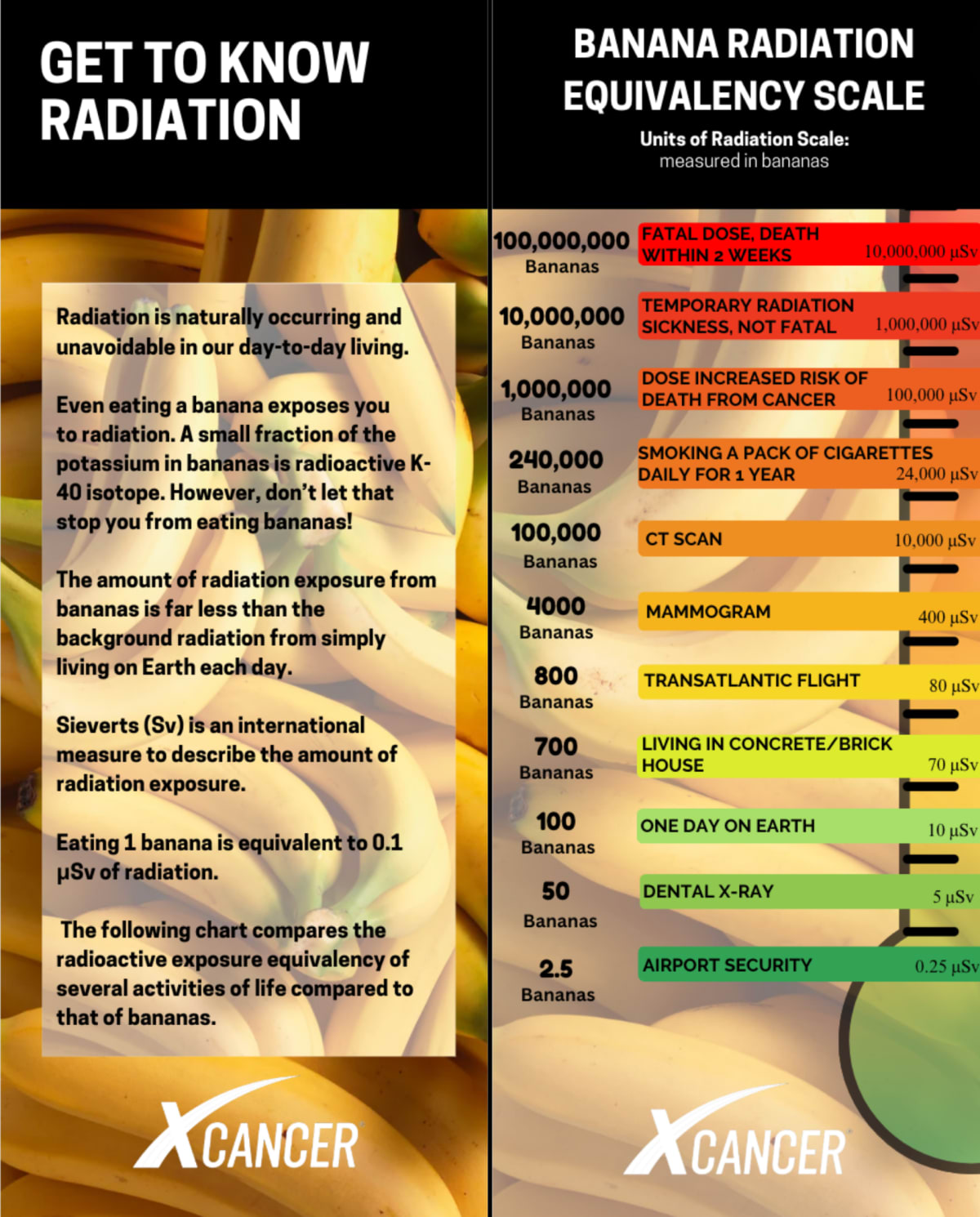
Education























.svg)















