
TheranosticTrials ecosystem
The Leading CRO in Radiopharmaceutical Trial Management & Beyond
PSI is the leading pivotal-stage radiopharmaceutical CRO, currently running over 40% of the industry’s registrational nuclear medicine trials. Having brought the first targeted theragnostic to market, PSI continues to pioneer clinical research in the rapidly expanding radiopharmaceutical sector:
- Continually expanding a network of sites with specialized radiopharmaceutical workforce
- Helping create new-to-RLT-research sites to address the demand and the limited infrastructure challenges
- Creating new, unique roles and programs to support the busy or RLT-naïve site teams
- Working with regulators in countries unfamiliar with radiopharmaceuticals to expedite review and approvals
- Collaborating with global manufacturing and supply chain partners — including academic and industry leaders like the University of Missouri and Xcancer — to help secure and scale isotope availability for next-generation therapies.

PSI Radiopharmaceutical Portfolio (as of August 2025)
PSI specializes in delivering diagnostic and theragnostic pivotal trials that require diverse geographies and complex logistics. PSI’s strength lies in seamlessly coordinating multidisciplinary site teams, ensuring regulatory and logistical readiness, specialized site support, and developing tailored recruitment strategies to meet the unique demands of radiopharmaceutical research.
- 177Lu
- 68Ga
- 225Ac
- 212Pb
- 64Cu
- 89Zr
- 18F
- Tc-99m
- 153Sm
- PSMA
- SSTRs
- FRs
- GRPR
- Integrin αvβ6
- SCFAs
- GPC3
- FAP
- Prostate cancer
- GEP-NETs
- NSCLC
- Osteoblastic metastatic bone cancer
- Hepatocellular carcinoma
- Pancreatic ductal adenocarcinoma
- Breast cancer
- Parkinsonian syndrome
- Surgical interoperative imaging
- Pain
- North America
- South America
- Europe, Western/Central/ Eastern
- Asia Pacific
PSI Radiopharmaceutical Expertise
- Radiopharm strategy team dedicated to developing tailored recruitment plans and training curriculum, organizing communication and logistics across multidisciplinary site teams, and supporting the project teams
- Radiopharmaceutical division that houses experts in the fields of nuclear medicine, imaging, dosimetry and physics, and nuclear pharmacy
- Over 20 board-certified in-house oncologists with radiopharm experience in clinical trials (and hiring)
- Over 40 oncologists on PSI’s Scientific Advisory Board, including radiologists and a neuroradiologist
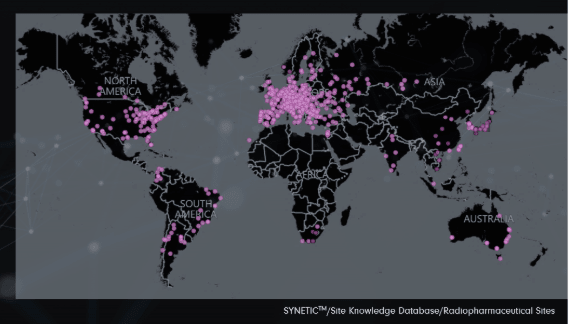
- Over 1,000 vetted radiopharm sites with clinical trial experience
Radiopharmaceutical Site Development Program
At the site level, our dedicated development program equips motivated research centers with the training, infrastructure, and regulatory support needed to participate in radiopharmaceutical trials. This dual focus expands global site capacity and stabilizes delivery logistics — giving sponsors a reliable, scalable framework for successful trial execution.
Our Global Network of Trusted Sites
With PSI, you’re never alone with these challenging trials. Get in touch with our team of experts today at psi-cro.com.
